
Disposablc Vacuum Sealing Drainage Dressing
Product Name
Disposable negative pressure drainage sponge
Model specification
According to the sponge material and size, there are three categories and 20 models.
Structure and composition
Disposable negative pressure drainage sponge
Model specification
According to the sponge material and size, there are three categories and 20 models.
Structure and composition
Disposable negative pressure drainage sponge consists of non-functional sponge (containing purified water to maintain the wetting state), medical film, cleaning tube, drainage tube, connectors, extension tubes, stopping the liquid clip and optional accessories suction cups, of which the medical film from the release paper, polyurethane (PU film, acrylic resin pressure-sensitive adhesive and polyethylene (PE) film composition. The products are supplied in a sterile state, sterilised by irradiation and are for single use.


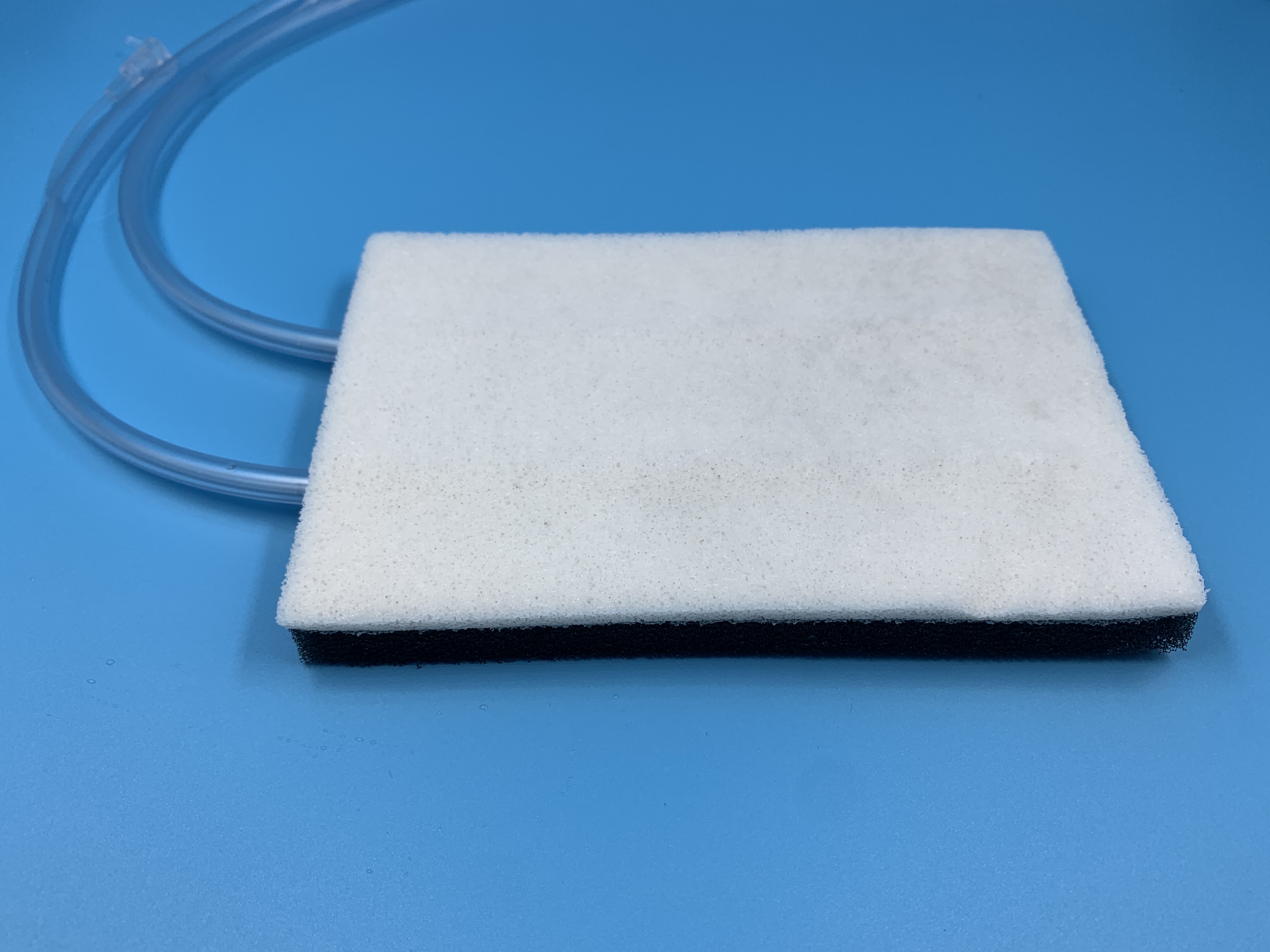
Main performance of the product
The non-functional sponge in the product should have no stains in appearance, no debris, and the aperture size is basically uniform; the film should be regular in shape, uniform in thickness, and no defects; the surface of the pipeline should be smooth, with no obvious sharp edges and burrs, and the inner lumen should be free of foreign matter and impurities; the bacterial endotoxin of the non-functional sponge should be ≤0.5EU/mg; the product should be sterile after irradiation.
Scope of application
Used for drainage of non-chronic wounds (e.g. post-surgical suture wounds, mechanical trauma, cut wounds, superficial II degree burn wounds, etc.).
Precautions and warnings
1. The use of this product should be operated by qualified professionals;
2. Check whether the package is intact before use. And confirm the packaging mark, production date, sterilisation expiry date, and use it within the sterilisation expiry date;
3. Avoid the contact between this product and organic solvents such as alcohol;
4. One-time use, prohibit repeated use;
5. The package is damaged, or found to be expired, foreign objects in the package or contaminated, prohibit the use;
6. Recommended use of negative pressure value of 10Kpa ~ 16Kpa, the requirements should not exceed 60Kpa;
7. The product should be handled in accordance with the medical waste method after use;
The non-functional sponge in the product should have no stains in appearance, no debris, and the aperture size is basically uniform; the film should be regular in shape, uniform in thickness, and no defects; the surface of the pipeline should be smooth, with no obvious sharp edges and burrs, and the inner lumen should be free of foreign matter and impurities; the bacterial endotoxin of the non-functional sponge should be ≤0.5EU/mg; the product should be sterile after irradiation.
Scope of application
Used for drainage of non-chronic wounds (e.g. post-surgical suture wounds, mechanical trauma, cut wounds, superficial II degree burn wounds, etc.).
Precautions and warnings
1. The use of this product should be operated by qualified professionals;
2. Check whether the package is intact before use. And confirm the packaging mark, production date, sterilisation expiry date, and use it within the sterilisation expiry date;
3. Avoid the contact between this product and organic solvents such as alcohol;
4. One-time use, prohibit repeated use;
5. The package is damaged, or found to be expired, foreign objects in the package or contaminated, prohibit the use;
6. Recommended use of negative pressure value of 10Kpa ~ 16Kpa, the requirements should not exceed 60Kpa;
7. The product should be handled in accordance with the medical waste method after use;
[Product storage and transport]
This product is a sterile medical device, in the process of transport and movement need to be held gently, avoid excessive collision and extrusion; avoid extreme temperatures; avoid exposure to dust and corrosive air; avoid exposure to excessive impact on the environment. Store in a cool and dry room with relative humidity not exceeding 80%, no corrosive odour and well ventilated.
This product is a sterile medical device, in the process of transport and movement need to be held gently, avoid excessive collision and extrusion; avoid extreme temperatures; avoid exposure to dust and corrosive air; avoid exposure to excessive impact on the environment. Store in a cool and dry room with relative humidity not exceeding 80%, no corrosive odour and well ventilated.

